My Lab
 Prof. Yu-Chen Hu
Prof. Yu-Chen Hu
Fellow, American Institute for Medical and Biological Engineering (AIMBE)
Fellow, Biomaterials Science and Engineering (FBSE)
Co-chair, TEB division, AFOB
Tsing Hua Chair Professor
Department of Chemical Engineering, National Tsing Hua University
Hsinchu, Taiwan
Dear AFOB members,
I would like to first thank Prof. Yoon-Mo Koo, Editor-in-Chief of AFOB Newsletter, to give me this opportunity to introduce my lab and research to all AFOB members. I received my BS degree in Chemical Engineering from National Taiwan University in 1992, PhD degree in Chemical Engineering from University of Maryland in 1999, and post-doc training at National Institutes of Health (USA) from 1999 to 2000. I started my faculty career as an assistant professor at National Tsing Hua University in 2000 and was promoted to associate professor in 2003, professor in 2007, distinguished professor in 2010 and Tsing Hua Chair Professor in 2019.
I have won the Asia Research Award, Outstanding Research Award twice (Ministry of Science and Technology, 2006, 2014), BEST Biochemical Engineering Achievement Award, Wu Ta-You Memorial Award, Jin Kai-Yin Award, Mao Gao-Wen Award, Outstanding Academia-Industry Research Award and Outstanding Young Investigator Award in Taiwan. I am a fellow of American Institute for Medical and Biological Engineering (AIMBE), as well as a Fellow Biomaterials Science and Engineering (FBSE), International Union of Societies for Biomaterials Science and Engineering.
I have served as the Program Chair of the Tissue Engineering International & Regenerative Medicine Society-Asia Pacific (TERMIS-AP) 2016 meeting, Conference co-chair of Asian Congress of Biotechnology (ACB meeting 2019) and the Vice President of Biotechnology and Biochemical Engineering Society of Taiwan. I am the TERMIS-AP council member, executive board member and co-chair of Tissue Engineering and Biomaterials (TEB) Division of AFOB. I currently serve as the associated editor of Current Gene Therapy and deputy editor of Journal of Taiwan Institute of Chemical Engineers. I was also the Coordinator of Chemical Engineering Division, Ministry of Science and Technology, Taiwan.
My research interests include vaccine development, gene therapy, tissue engineering, cancer therapy and synthetic biology. I have developed the enterovirus 71 (EV71) vaccine based on virus-like particle technology and also pave a new avenue to the use of baculovirus as a novel vector for gene delivery, regenerative medicine and cancer therapy. I also combined burgeoning CRISPR technology and synthetic biology for the metabolic engineering of microorganisms and stem cells for production of bio-derived chemicals and tissue regeneration. In particular, I have recently developed various CRISPR activation (CRISPRa) and CRISPR interference (CRISPRi) and CRISPRai platforms to modulate stem cell differentiation so as to improve regeneration of bones and nerves.
1. Vaccine development
● Development of virus-like particle (VLP) vaccines for enterovirus 71:
I have pioneered the development of enterovirus 71 virus particles (VLP) vaccine. My seminal paper captured widespread attention and extensive media coverage, which is not only cited more than 282 times (WOS 170 times), but also attracts GlaxoSmithKline, Merck and Pfizer and other world-class pharmaceutical companies to negotiate cooperation with me. The Department of Health also grants me a special funding of  1 million USD to develop the vaccine. This is the world's first genetically engineered enterovirus 71 vaccine, which has become the gold standard for the development of enterovirus VLP vaccines. My VLP technology has licensed to Medigen Biologicals for a licensing fee of
1 million USD to develop the vaccine. This is the world's first genetically engineered enterovirus 71 vaccine, which has become the gold standard for the development of enterovirus VLP vaccines. My VLP technology has licensed to Medigen Biologicals for a licensing fee of 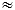 100,000 USD and ensuing royalty.
100,000 USD and ensuing royalty.
● Development of self-amplifying RNA vaccine:
My lab has also developed the self-amplifying RNA vaccine against a pig virus. The self-amplifying RNA vaccine can be delivered into animal models and induce potent and faster immune responses in animals that overcome the drawbacks of current vaccines.
2. Gene therapy for tissue engineering and cancer therapy
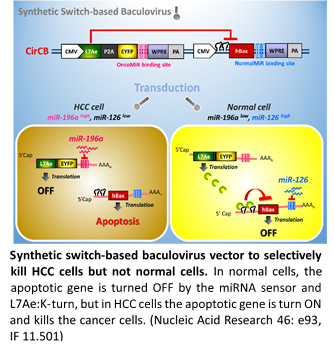 Baculovirus is an insect virus that is harmless to humans but can efficiently deliver genes (transduce) into a wide variety of mammalian cells. Its application in gene delivery into mammalian cells is a relatively new area over the last decade. I have developed novel hybrid baculovirus vectors, which allow for efficient transgene excision off baculovirus genome and existence of transgene cassette as an episomal DNA minicircle, thereby significantly prolonging the transgene expression. I was also invited to write a review paper published in Nature Protocols. We have also recently exploited synthetic biology for the development of gene therapy. We used an L7Ae/K-turn repression module and miRNA binding site to build a synthetic circuit. We used the circuit to control anticancer gene expression and incorporated this device into baculovirus so that the baculovirus can selectively kill cancer cells, without harming normal cells.
Baculovirus is an insect virus that is harmless to humans but can efficiently deliver genes (transduce) into a wide variety of mammalian cells. Its application in gene delivery into mammalian cells is a relatively new area over the last decade. I have developed novel hybrid baculovirus vectors, which allow for efficient transgene excision off baculovirus genome and existence of transgene cassette as an episomal DNA minicircle, thereby significantly prolonging the transgene expression. I was also invited to write a review paper published in Nature Protocols. We have also recently exploited synthetic biology for the development of gene therapy. We used an L7Ae/K-turn repression module and miRNA binding site to build a synthetic circuit. We used the circuit to control anticancer gene expression and incorporated this device into baculovirus so that the baculovirus can selectively kill cancer cells, without harming normal cells.
● Exploiting gene delivery for regenerative medicine:
Healing of massive bone defects remains a challenge for orthopedic surgeons. We have developed novel hybrid baculovirus vector for long-term transgene expression in stem cells and pioneered the use of hybrid baculovirus to engineer adipose-derived stem cells (ASCs) or bone marrow-derived mesenchymal stem cells (BMSCs) to deliver various genes including growth factors (e.g. BMP-2, VEGF, SDF-1, BMP-6, TGF-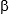 3), microRNA, microRNA sponge to stimulate regeneration of bone or cartilage in massive defect models. These studies collectively prove the effectiveness and safety of this approach and pave a new avenue to exploiting novel hybrid baculovirus vectors for tissue regeneration.
3), microRNA, microRNA sponge to stimulate regeneration of bone or cartilage in massive defect models. These studies collectively prove the effectiveness and safety of this approach and pave a new avenue to exploiting novel hybrid baculovirus vectors for tissue regeneration.
Baculovirus-engineered adipose-derived stem cells (ASC) repair bone defects at the calvaria and femora. (a) The rabbit ASCs (rASCs) were co-transduced with baculoviruses expressing appropriate transgenes (e.g. BMP-2 and microRNA), seeded to scaffolds and transplanted into defects. (b-e) The baculovirus-engineered cells repair the large defects at the calvaria and femora. (Biomaterials, 2017, 124:1; Biomaterials, 2016, 74:155; Biomaterials, 2015, 50:98; Biomaterials 2012, 33: 3682).
● Cancer therapy
We also combined baculovirus-mediated gene delivery with cancer therapy. we have combined the Sleeping Beauty-based hybrid baculovirus vector and miRNA to combat hepatocellular carcinoma (HCC). We use the baculovirus to integrate the expression cassettes of miR-122 and miR-151 sponge, so as to simultaneously enhance miR-122 expression while attenuating miR-151 expression. By concomitant regulation of these 2 miRNAs, the hybrid baculovirus mitigates the proliferation and migration of aggressive HCC cells. Injection of the hybrid baculovirus into HCC tumor model effectively suppresses liver cancer progression and metastasis. This work was published in Molecular Therapy and soon became a High Cite paper.
In addition, we have used the hybrid baculovirus vector to express long non-coding RNA (lncRNA) PTENP1 as a miRNA decoy to enhance the levels of tumor suppressor protein PTEN. Injection of the baculovirus vector inhibits liver cancer progression and metastasis. Thanks to the novel findings, this paper was published in Biomaterials and became a High Cite paper.
3. Application of nanomaterials in biomedical engineering
Graphene and graphene oxide (GO) are promising nanomaterials for diverse applications. I have first uncovered that functionalizing glass substrate with graphene or GO can guide the differentiation of induced pluripotent stem cells (iPSCs) toward specific lineage, hence implicating the potential applications of GO for iPSCs culture and differentiation modulation. This paper was published in Biomaterials and is a High Cite paper (327 citations).
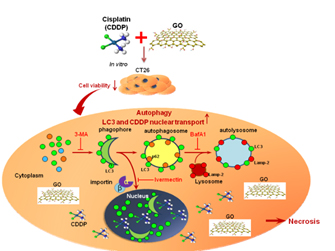
GO as a new class of anticancer agent and chemosensitizer. GO can induce autophagy and TLR signaling in cancer cells. Combination of GO and cisplatin alters autophagic flux and promotes nuclear trafficking of cisplatin, hence potentiating the ability to kill different cancer cells. (Biomaterials 2012, 33: 6559; Biomaterials 2015, 40: 12)
We further uncovered that GO can elicit autophagy in macrophage and activate toll-like receptors 4 and 9 (TLR-4, TLR-9) and downstream signaling pathway, which play critical roles in innate immune responses. This study first unveiled that nanomaterials such as GO can provoke TLR-associated inflammatory responses and is a High Cite paper (162 citations). We next show that injection of GO into colon cancer mouse models elicits autophagy and tremendously boosts TLR-associated anticancer immune responses, rendering GO a promising immunomodulatory and anticancer agent.
4. CRISPR-based genome editing and synthetic biology
CRISPR is a versatile RNA-guided genome engineering tool developed in 2012 and is recognized by 2020 Nobel Prize in Chemistry. I started the CRISPR and synthetic biology research in 2013 and exploit these technologies in regenerative medicine and microbial metabolic engineering. I use synthetic biology principles to design synthetic switches and exploit CRISPR-derived technology to enhance or inhibit gene expression, thereby regulating the differentiation state of cells. I have published 18 papers in this field, including 10 benchmark papers, 1 High Cite paper, and a Nature Biomedical Engineering (IF 18.952) review.
● Applications in metabolic engineering and production of bio-derived chemicals:
I have leveraged CRISPR technology for microbial genome editing, regulation of metabolic pathways and synthesis of bio-derived chemicals. CRISPR relies on Cas9 nuclease and guide RNA to target and trigger DNA double strand break at pre-determined chromosomal site. We optimize the process of CRISPR-Cas9 system in E. coli and successfully integrate gene cassettes of up to 10 kb into the chromosome, thus overcoming the size limit ( 3.5 kb) of current techniques. This paper was published in 2017 and soon became a High Cite paper.
3.5 kb) of current techniques. This paper was published in 2017 and soon became a High Cite paper.
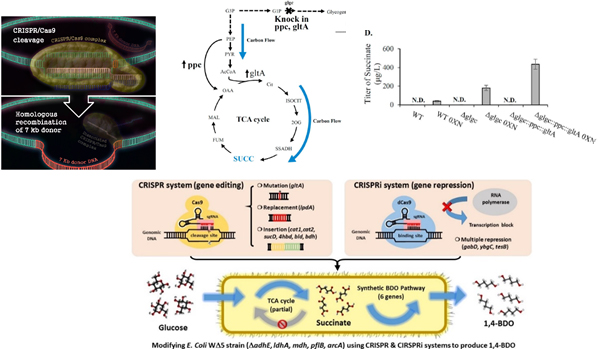
Development of CRISPR/CRISPRi to regulate metabolic pathways in microorganisms for the production of bio-derived chemicals. (a) Use of CRISPR to integrate large DNA fragments into the E. coli chromosome. (b-c) Use of CRISPR-Cas9 system to rewire the metabolic pathways in cyanobacteria to enhance the succinate production. (d) Combining CRISPR and CRISPRi for simultaneous gene editing and gene regulation in E. coli to introduce and regulate the synthetic pathway for the production of 1,4 butanediol. (Biotechnol. Bioeng. 2017, 114: 172. Metabolic Engineering 2016, 38: 293; ACS Synthetic Biology 2017, 6:2350-2361; Biotechnol. Bioeng. 2019, 116: 1066-1079; ACS Synth Biol. 2020. 9: 1138-1149).
Cyanobacteria are photosynthetic microorganism capable of utilizing CO2 as the carbon source. We exploited the CRISPR system to integrate large gene cassette into cyanobacterial chromosome and significantly accelerate the process of obtaining stable strain. In addition to CRISPR, we developed CRISPRi system to knockdown gene expression in cyanobacteria and E. coli. We further combine CRISPR and CRISPRi to simultaneously edit, swap and insert large synthetic pathway genes into the bacterial chromosome and suppress gene expression to rewire the metabolic pathways for 1,4 butanediol production. We have also used CRISPR to rapidly integrate genes into the polyploid Pseudomonas putida S12 so as to modulate the metabolic pathway for FDCA production.
● Applications in stem cell engineering and regenerative medicine:
CRISPR activation (CRISPRa) is a tool that exploits catalytically dead Cas9 (dCas9), sgRNA and transcription activator for gene activation. We developed a hybrid baculovirus vector to harbor and deliver the CRISPRa system for multiplexed activation of 3 endogenous neurotrophic factor genes (BDNF, GDNF and NGF). The hybrid baculovirus is able to functionalize the adipose-derived stem cells (ASC) sheets. Implantation of the CRISPRa-engineered ASC sheets into sciatic nerve injury sites in rats induced successful nerve repair and restoration of nerve functions.
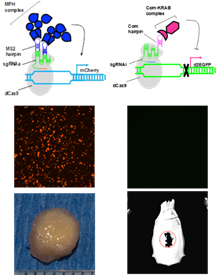
Development of CRISPRai system for simultaneous gene activation and inhibition for tissue regeneration. The CRISPRai module comprised the dCas9 regulator, MCP-p65-HSF1 (MPH) as the activation complex and Com-KRAB as the repression complex. We also expressed sgRNAi and sgRNAa that recruit the repression complex and activation complex, respectively. The CRISPRai system was able to to simultaneously activate Sox9 gene and inhibit PPAR-, thus enhancing chondrogenesis of stem cells and formation of cartilage in 3D culture. Implantation of the CRISPRai-engineered cells improved calvarial bone healing. (Nucleic Acids Research 47: e74 (IF 11.501)
We have also used CRISPRi to suppress Noggin gene, upon co-expression of BMP-2, so as to enhance the osteoinductive effects of BMP-2. The CRISPRi-mediated Noggin suppression improved the BMP-2-induced ASC differentiation and augmented the bone regeneration, after implantation.
To simultaneously promote chondrogenesis and repress adipogenesis of stem cells, we exploited the RNA scaffold concept and built a CRISPRai system that comprised inactive Cas9 (dCas9), 2 fusion proteins as activation/repression complexes and 2 single guide RNA (sgRNA) as scaffolds for recruiting activator (sgRNAa) or inhibitor (sgRNAi). This CRISPRai system allows us to simultaneously activate the Sox9 gene governing chondrogenesis and inhibit PPAR- gene governing adipogenesis. This CRISPRai thus enables concurrent stimulation of chondrogenesis and repression of adipogenesis of stem cells. The CRISPRai system delivered by baculovirus stimulates chondrogenesis and represses adipogenesis of stem cells in 2D culture and promotes the formation of engineered cartilage in 3D culture. Implanting the engineered stem cells further improves the calvarial bone healing. This study paves a new avenue to translate the CRISPRai technology to regenerative medicine.
gene governing adipogenesis. This CRISPRai thus enables concurrent stimulation of chondrogenesis and repression of adipogenesis of stem cells. The CRISPRai system delivered by baculovirus stimulates chondrogenesis and represses adipogenesis of stem cells in 2D culture and promotes the formation of engineered cartilage in 3D culture. Implanting the engineered stem cells further improves the calvarial bone healing. This study paves a new avenue to translate the CRISPRai technology to regenerative medicine.
Acknowledgements
I would like to acknowledge the long-term support of Ministry of Science and Technology, Taiwan. In particular, my special thanks go to all students and post-doc who work so hard on these challenging projects.

Selected papers (2017-2021) * Corresponding author IF: 2019 Impact factor
1. Nguyen, TKN, Chang, Y.-H., Truong, A.V., Hsu, M.-N., Pham, NN, Chang, C.-W., Wu, Y.-H., Chang, Y.-H., Li, H., Hu, Y.-C.*. 2021. CRISPR activation of long non-coding RNA DANCR promotes bone regeneration. Epub ahead of print. Biomaterials. https://doi.org/10.1016/j.biomaterials.2021.120965 (IF 10.317).
2. Klionsky, D., Abdel-Aziz, A.K., .Hu, Y.-C., et al. 2021 Feb. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy. 1-382. (IF 9.770).
3. Hsu, M.-N., Yu, F.-J., Chang, Y.-H., Huang, K-L., Pham, N. N., Troung, A.V., Lin, M.-W., Nguyen, N.T.K., Hwang, S.-M., Hu, Y.-C.* 2020 Sep. CRISPR interference-mediated Noggin knockdown promotes BMP2-induced osteogenesis and calvarial bone healing. Biomaterials. 252: 120094. (IF 10.317).
4. Hsu, M.-N, Huang, K-L., Yu, F.-J., Lai, P.-L., Troung, A.V., Lin, M.-W., Nguyen, N.T.K., Shen, C.-C., Hwang, S.-M., Chang, Y.-H., Hu, Y.-C.* 2020 Feb. Co-Activation of endogenous Wnt10b and Foxc2 by CRISPR activation enhances BMSCs osteogenesis and promotes calvarial bone regeneration. Molecular Therapy 28: 441-451 (IF 8.986).
5. Hsu, M-N., Chang, Y.-H., Truong, V. A., Nguyen, N.T.K., Hu, Y.-C.* 2019 Dec. CRISPR technology for stem cell engineering and regenerative medicine. Biotechnology Advances 37:107447. (IF 10.744).
6. Hsu, M.-N., Hu, Y.-C.*. Local magnetic activation of CRISPR. 2019 Feb. Nature Biomedical Engineering. 3: 83-84. (IF 18.952).
7. Truong, V. A., Hsu, M-N., Nguyen, N.T.K., Lin, M-W., Shen, C.-C., Lin, C.-Y., Hu, Y.-C.* 2019. July. CRISPRai for simultaneous gene activation and inhibition to promote stem cell chondrogenesis and calvarial bone regeneration. Nucleic Acids Research. 47: e74 (IF 11.501).
8. Shen, C.-C., Hsu, M.-N., Chang, C.-W., Lin, M.-W., Hu, Y.-C*. 2019 Feb. Synthetic switch to minimize CRISPR off-target effects by self-restricting Cas9 transcription and translation. Nucleic Acids Research. 47: e13 (IF 11.501).
9. Hsu, M-N., Liao, H.-T., Truong, V. A., Huang, K.-L., Yu, F.-J., Chen, H.-H., Nguyen, N.T.K., Makarevich P., Parfyonova, Y., Hu, Y.-C.* 2019 Aug. CRISPR-based activation of endogenous neurotrophic genes in adipose stem cell sheets to stimulate peripheral nerve regeneration. Theranostics 9: 6099-6111 (IF 8.579).
10. Lin, M.-W., Tseng, Y.-W., Shen, C.-C., Hsu, M.-N., Hwu, J.-R., Chang, C.-W., Yeh, C.-J., Chou, M.-Y., Wu, J.-C., Hu, Y.-C.*. 2018, Sep. Synthetic switch-based baculovirus for transgene expression control and selective killing of hepatocellular carcinoma cells. Nucleic Acids Research. 46: e93 (IF 11.501).
11. Lin, K.-C., Lin, M.-W., Chen, G.-Y., Chao, Y.-C., Tuan, H.-Y., Chiang, C.-S., Hu, Y.-C.*. 2018 March. Graphene oxide chemosensitizes cancer cells to cisplatin by inducing early autophagy events, promoting nuclear trafficking and necrosis. Theranostics 8: 2477-2487 (IF 8.579).
12. Masimukku, S., Hu, Y.-C., Lin, Z.-H., Chan, S.-W., Chou, T.-M., Wu, J.-M. 2018 April. High efficient degradation of dye molecules by PDMS embedded abundant single-layer tungsten disulfide and their antibacterial performance. Nano Energy. 46: 338-346. (IF 16.602).


